USC investigating prominent neuroscientist
A 113-page dossier accuses Berislav Zlokovic of data manipulation in studies.
A 113-page dossier accuses Berislav Zlokovic of data manipulation in studies.
USC has launched an investigation into longtime University professor Berislav Zlokovic’s work, following accusations that the well-known neuroscientist manipulated data to fraudulently promote the efficacy of an experimental drug.
A group of external scientists sent the National Institutes of Health a 113-page dossier calling attention to evidence indicating that the drug, which has received significant federal financial backing and FDA Fast Track status to treat ischemic stroke patients, might have increased rather than decreased deaths in sampling.
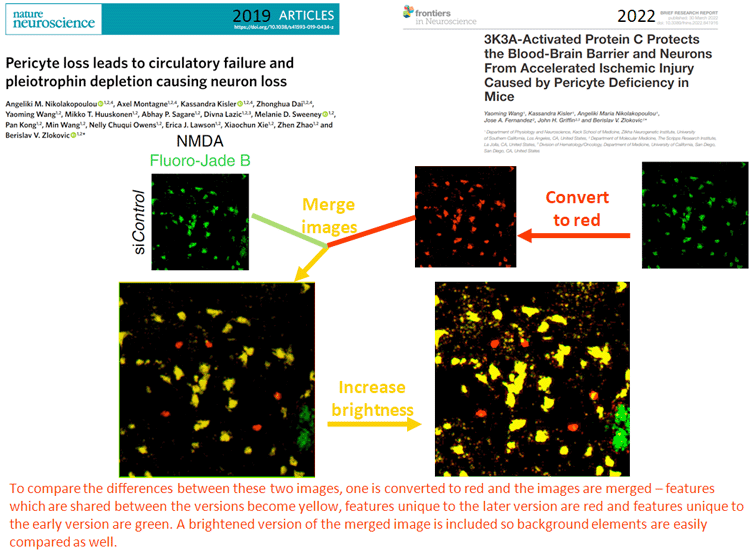
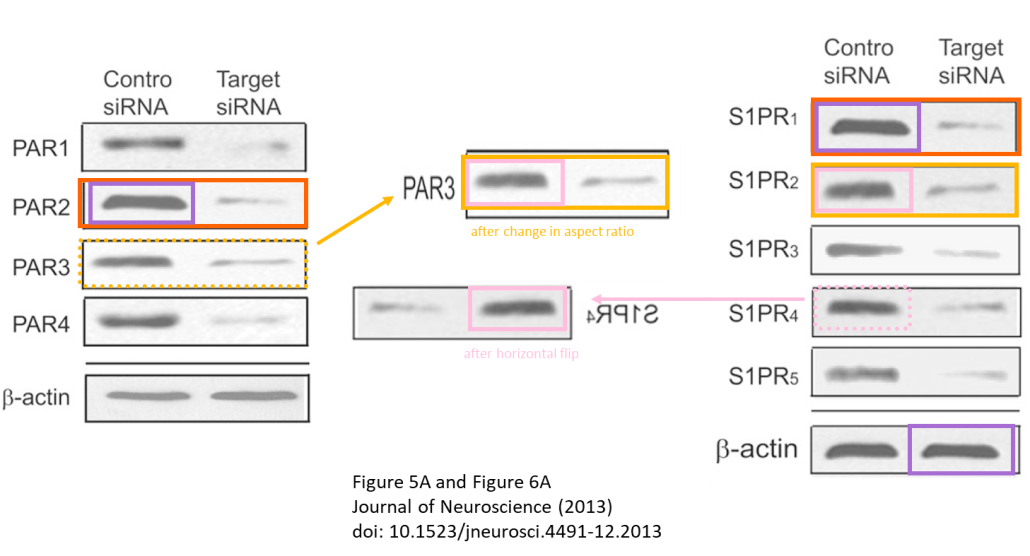
It also detailed examples of photos that are alleged to have been doctored by Zlokovic’s lab going back almost two decades.
Matthew Schrag, the lead whistleblower who contributed to the dossier and a neuroscientist at Vanderbilt University, described “a fairly pervasive pattern of unexpected anomalies over about 20 years.”
“The pattern of anomalies that we’re looking at is alarming,” Schrag said in an interview with the Daily Trojan. “It’s a fact pattern that is hard to explain away … and I think that it’s appropriate to start an investigation.”
Over the last five years, a handful of scientists began scrutinizing Zlokovic’s work on PubPeer, a website where scientists publish concerns about scientific misconduct. Zlokovic has published 44 papers on the drug — known as 3K3A-APC — alone, claiming that it showed promise of reducing brain cell death, bleeding and inflammation in patients after they experienced a life-threatening ischemic stroke.
Earlier this year, Schrag joined fellow researchers in examining Zlokovic’s work more closely, although independently. Among that group is Elisabeth Bik, a science integrity consultant, who shed light on some of the issues the researchers found in Zlokovic’s papers.
“We found several types of image problems in these papers,” Bik said. “[In] some of these, an image has been reused in different studies to represent a different experiment, but it’s the same photo or overlapping photos. And then we also found some photos that appear to have elements either added or removed.”
In examples that were shown to the Daily Trojan, elements of photographs appear to have been copied, pasted and rotated onto others in order to give the impression that 3K3A-APC was more effective in treating patients. In another, an image of a neuron appears to have been incorrectly superimposed into an area where a blood vessel already exists. Photographs also appear to have been used to prove the results of different experiments in 2004 and 2009.
“It’s really hard to compare the images across each other when you have different experimental conditions, for example,” Bik said. “So it seemed that there was in some of these photos an intention to mislead.”

In the dossier, the whistleblowers also highlight evidence that, during Zlokovic’s Phase II trial, six of the 66 stroke patients who were administered 3K3A-APC died within a week after receiving the drug, compared to only one out of the 44 members of the placebo group. Patients who received the real drug were also apparently more likely to become disabled 90 days after receiving the drug, compared to those who received the placebo.
Schrag said there were three major issues with the results, two of them being the number of deaths and the symptoms shown by patients.
The third, he said, was “a skew in the randomization strategy” where placebo patients got their standard stroke treatments much later than patients in the treatment group. Although Schrag did not pinpoint this to misconduct, he described it as one of the three “red flags.”
Treating stroke patients is considered by doctors as a time-sensitive matter. Both Bik and Schrag took issue with the way that stroke survivors included in the sampling were given the pressure of deciding whether to choose an experimental drug to help with their treatment with the false pretenses that the drug was developed under.
In anonymous interviews also published by Science Magazine on Nov. 13, four former members of Zlokovic’s lab claimed that the professor altered images in journal articles and forced his research staff to change their data in order to present the results he desired.
“It’s maybe not the senior researcher who is changing these images, but it could be an atmosphere of bullying and high demand put on junior researchers,” Bik said. “I feel that very often these senior researchers will throw the junior persons under the bus.”
Zlokovic’s research has received substantial federal backing up until now. In June 2020, the U.S. Food and Drug Administration gave 3K3A-APC Fast Track status because of its potential to treat a serious condition. Later, in May 2022, the National Institute of Neurological Disorders and Stroke awarded a $30 million grant to Zlokovic’s research team at the Keck School of Medicine to conduct a Phase III clinical trial using 3K3A-APC.
Knowing that $4 million of the $30 million grant has already been spent on developing the drug, Bik said that “you would hope that he might be able to give it back.”
“But it would depend on the outcome of the investigation,” Bik said. “That will take months and probably years and so in the meantime, I assume the research is still ongoing … by the time the investigations are done, I’m expecting this to take at least one if not two years, or even longer than I assume all the grants by them have run out already.”
Zlokovic has received roughly $93 million in NIH funding during his career, according to Science Magazine. Under Zlokovic’s leadership, USC’s Keck institute has expanded to more than 30 labs and grown its annual funding more than tenfold, exceeding $39 million in 2022. According to USC, Zlokovic has added at least $28 million from private sources.
“In general, it makes me frustrated if I see signs of what could be misconduct because there’s just so much competition about scientific grants,” Bik said. “The second frustration I have is where people base their research on the work of others. So, if these papers are published, other people might base their research on these papers and might have trouble reproducing these papers.”
Zlokovic has acted as chair of the Department of Physiology & Neuroscience at the Keck School of Medicine of USC since 2011. Zlokovic also serves as the Mary Hayley and Selim K. Zilkha Chair in Alzheimer’s Disease Research and the director of the Zilkha Neurogenetic Institute.
“Winning lots of great grants and having a big lab with lots of people working [for] you for many years — that is probably by most people’s definition of [a] successful scientist,” Bik said.
The University declined to provide comment on whether or not Zlokovic will maintain his leadership positions while the investigation into his conduct is underway.
“As one of the nation’s leading institutions for innovative, impactful discovery, USC takes any allegations relating to research integrity seriously,” the University wrote in a statement to the Daily Trojan Tuesday. “Consistent with federal regulations and USC policies, the university forwards any such allegations to its Office of Research Integrity for careful review.”
Although the Daily Trojan asked for details about what the University might do with the money it has so far received for Zlokovic’s research, the University said in its statement that it could not comment because “under USC policy, this review is required to be confidential.”
Zlokovic did not respond to a request for comment by the Daily Trojan, and neither did his private company, ZZ Biotech, through which the drug is being developed.
His attorney was also approached for comment but did not respond, although in his statement to Science he wrote that Zlokovic “is committed to fully cooperating” with the USC inquiry.
This marks another moment in a period where academic misconduct, particularly at the university level, is under heightened scrutiny. In July, the president of Stanford University Marc Tessier-Lavigne resigned after it was found that he had authored 12 reports that contained falsified information.
“I think in a broader sense, the research misconduct is a bigger problem than we want to give it credit for,” Schrag said. “Even if it’s semi-uncommon, I don’t think we can say it’s rare anymore … [and] I think that we need to address it.”
We are the only independent newspaper here at USC, run at every level by students. That means we aren’t tied down by any other interests but those of readers like you: the students, faculty, staff and South Central residents that together make up the USC community.
Independence is a double-edged sword: We have a unique lens into the University’s actions and policies, and can hold powerful figures accountable when others cannot. But that also means our budget is severely limited. We’re already spread thin as we compensate the writers, photographers, artists, designers and editors whose incredible work you see in our daily paper; as we work to revamp and expand our digital presence, we now have additional staff making podcasts, videos, webpages, our first ever magazine and social media content, who are at risk of being unable to receive the support they deserve.
We are therefore indebted to readers like you, who, by supporting us, help keep our paper daily (we are the only remaining college paper on the West Coast that prints every single weekday), independent, free and widely accessible.
Please consider supporting us. Even $1 goes a long way in supporting our work; if you are able, you can also support us with monthly, or even annual, donations. Thank you.
This site uses cookies. By continuing to browse the site, you are agreeing to our use of cookies.
Accept settingsDo Not AcceptWe may request cookies to be set on your device. We use cookies to let us know when you visit our websites, how you interact with us, to enrich your user experience, and to customize your relationship with our website.
Click on the different category headings to find out more. You can also change some of your preferences. Note that blocking some types of cookies may impact your experience on our websites and the services we are able to offer.
These cookies are strictly necessary to provide you with services available through our website and to use some of its features.
Because these cookies are strictly necessary to deliver the website, refusing them will have impact how our site functions. You always can block or delete cookies by changing your browser settings and force blocking all cookies on this website. But this will always prompt you to accept/refuse cookies when revisiting our site.
We fully respect if you want to refuse cookies but to avoid asking you again and again kindly allow us to store a cookie for that. You are free to opt out any time or opt in for other cookies to get a better experience. If you refuse cookies we will remove all set cookies in our domain.
We provide you with a list of stored cookies on your computer in our domain so you can check what we stored. Due to security reasons we are not able to show or modify cookies from other domains. You can check these in your browser security settings.
These cookies collect information that is used either in aggregate form to help us understand how our website is being used or how effective our marketing campaigns are, or to help us customize our website and application for you in order to enhance your experience.
If you do not want that we track your visit to our site you can disable tracking in your browser here:
We also use different external services like Google Webfonts, Google Maps, and external Video providers. Since these providers may collect personal data like your IP address we allow you to block them here. Please be aware that this might heavily reduce the functionality and appearance of our site. Changes will take effect once you reload the page.
Google Webfont Settings:
Google Map Settings:
Google reCaptcha Settings:
Vimeo and Youtube video embeds:
The following cookies are also needed - You can choose if you want to allow them:
